Abstract:
Objective To investigate the feasibility and clinical value of transaxillary endoscopic assisted subcutaneous glandectomy with nipple and areolar preservation plus breast reconstruction with anterior breast prosthesis at stage Ⅰ.
Methods The clinical data of 32 women with breast cancer who underwent transaxillary endoscopic assisted papillary areolar subcutaneous glandectomy plus stageⅠbreast reconstruction with breast prosthesis were retrospectively analyzed from January to December 2022. SPSS 22.0 statistical software was used to analyze the data. Perioperative indicators, Breast-Q scale, Scar-Q scale and other measurement data were represented by (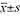 ). Paired sample t test was used for intra-group comparison. The statistical data of postoperative complications were shown as [cases (%)] and χ2 test was used. P < 0.05 was considered statistically significant.
). Paired sample t test was used for intra-group comparison. The statistical data of postoperative complications were shown as [cases (%)] and χ2 test was used. P < 0.05 was considered statistically significant.
Results All 32 patients successfully completed the axillary endoscopic assisted subcutaneous gland resection with preserving nipple and areolae plus stageⅠbreast prosthesis reconstruction. The operation time was (138.5±20.7) min, the intraoperative blood loss was (49.6±24.3) ml, and the number of sentinel lymph nodes was (3.0±1.0). The drainage time of the operative area was (7.3±1.5) d, the total drainage volume of the operative area was (504.8±19.6) ml, and the postoperative hospital stay was (6.3±1.2) d, and the incision margin of the postoperative pathological specimens of all patients were negative. The excellent and good rate of postoperative cosmetic effect evaluation was 93.8% (30/32). The results of Breast-Q score showed that the scores of chest wall status, sexual life and social psychological status at 1 and 3 months after surgery had statistical significance compared with those before surgery (P<0.05). The results of SCAR-Q score showed that the satisfaction of Scar appearance gradually increased with time, and the score increased to (66.1±6.2) points 6 months after surgery. Prosthesis displacement occurred in 1 case (3.1%), subcutaneous effusion in 2 cases (6.3%), and local necrosis of skin flap in 2 cases (6.3%), but there were no complications such as incision infection, incision dehiscence, postoperative bleeding, chest wall pain, capsular contracture, etc., and no local recurrence, distant metastasis, or death occurred in all patients during follow-up.
Conclusion Axillary endoscopic assisted subcutaneous gland resection with nipple and areolar preservation + breast reconstruction with anterior breast prosthesis in stageⅠis safe and feasible, and has obtained good radical, cosmetic and satisfactory results, which can be widely used in clinical practice.
Key words:
Breast Neoplasms,
Endoscopes,
Mastectomy, Modified Radical,
Breast Implantation,
Mammaplasty
Hong Liu, Pin Wang, Bin Wang, Jiechao Ren, Wenjie Zhang, Jian Wu, Ying Liu. Transaxillary endoscopic assisted subcutaneous glandectomy preserving nipple and areola + Stage Ⅰbreast reconstruction with anterior breast prosthesis[J]. Chinese Journal of Operative Procedures of General Surgery(Electronic Edition), 2024, 18(04): 419-422.